Details of the Drug
General Information of Drug (ID: DMZIUQ2)
| Drug Name |
4-DAMP
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
4-Diphenylacetoxy-1,1-dimethylpiperidinium; CHEMBL168067; 81405-11-0; CHEBI:73467; CHEMBL76897; 4-Damp methobromide; C21H26NO2; Tocris-0482; Lopac-D-104; AC1Q60WY; AC1L1C4C; 4-[(diphenylacetyl)oxy]-1,1-dimethylpiperidinium; Lopac0_000407; GTPL307; SCHEMBL2730650; 1952-15-4 (iodide); CTK3E9884; DTXSID70231086; 4-DAMP(1+); ZINC2555356; BDBM50176065; CCG-204500; NCGC00024611-02; NCGC00015304-04; NCGC00015304-03; NCGC00163244-01; NCGC00024611-01; NCGC00015304-02; NCGC00015304-01; LS-177624; N,N-dimethyl-4-(diphenylacetoxy)piperidinium
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
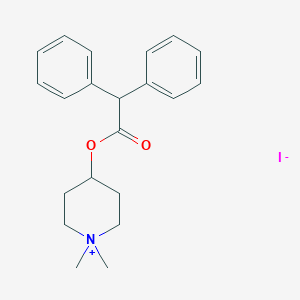 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight | 451.3 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||
| Rotatable Bond Count | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
